Company Certification
admin2025-08-31T07:51:08+00:00Proudly Registered for Trusted Quality
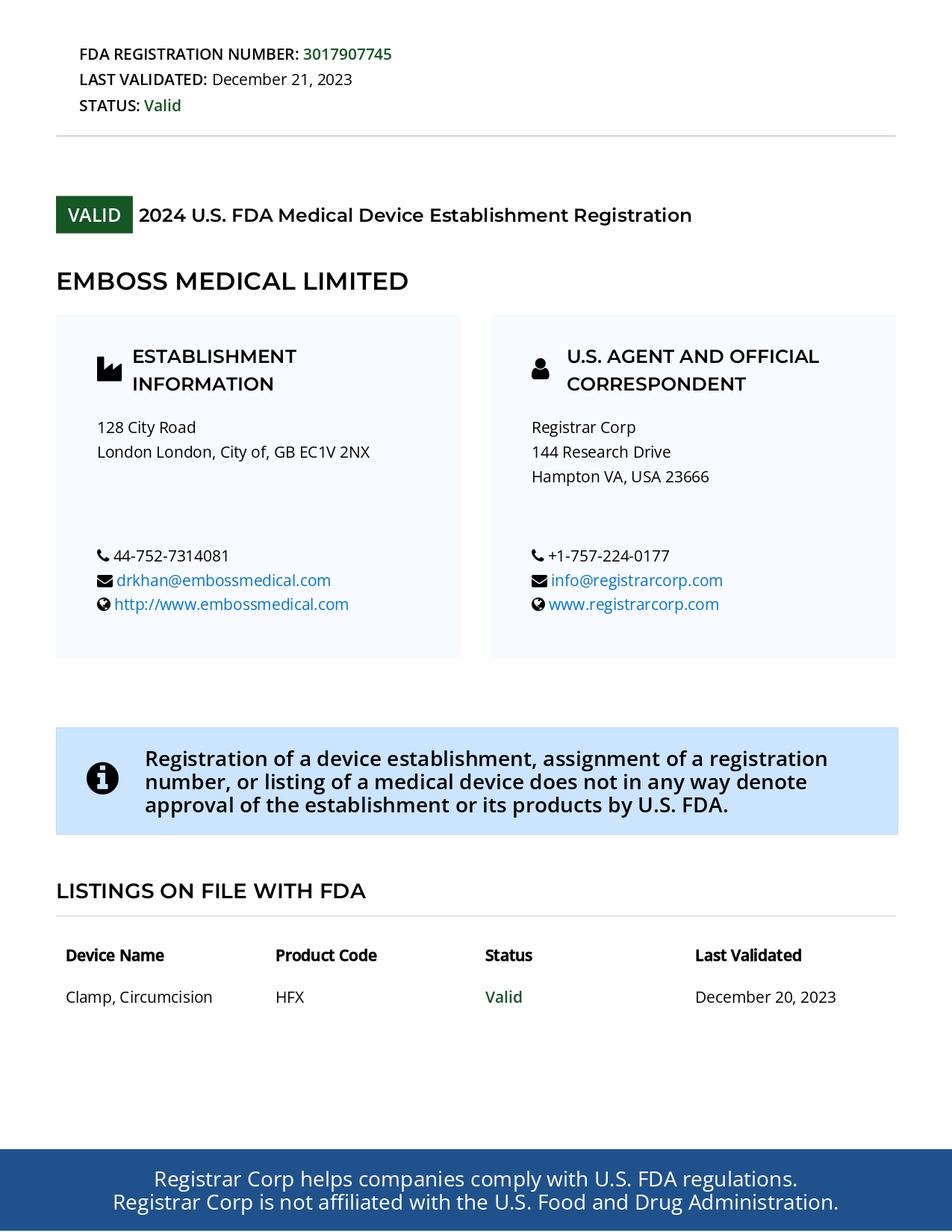
Authorities Registration: Our Commitment to Quality & Care
Our company takes pride in being FDA-registered, ensuring that our disposable circumcision devices meet the highest standards of safety, quality, and compliance. This recognition reflects our commitment to adhering to strict regulatory guidelines and delivering products that healthcare providers can trust. Through FDA registration, we guarantee that every device is manufactured under rigorous quality controls, sterile conditions, and in alignment with global medical standards. Our dedication to compliance and excellence reinforces our mission to provide safe, reliable, and innovative solutions for circumcision procedures worldwide.
We provide Advance Circumplast Devices
Most Innovative Circumcision Device For Male Children
Contact Us
info@novadiensurgical.com
+1 (413) 275-7616